The Baghdad Battery: Ancient Power Source or Medical Device? A Skeptical Electrochemical Analysis
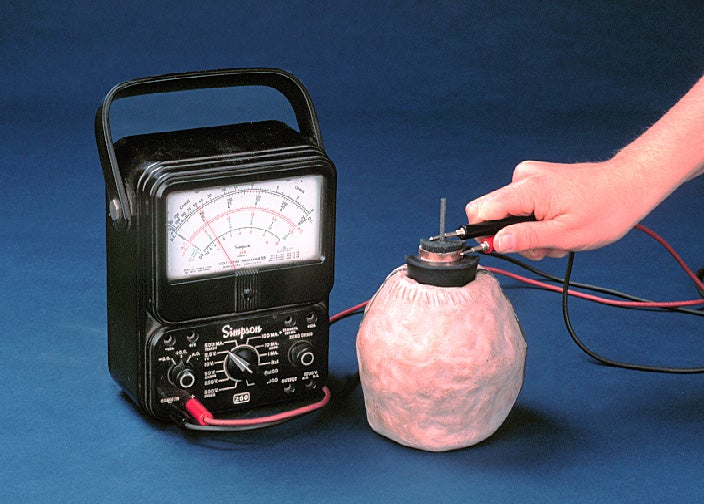
The Baghdad Battery, a collection of artifacts dating back to Mesopotamia (present-day Iraq), has captivated imaginations for decades. Is it evidence of ancient electrical knowledge, a forgotten technology capable of powering unknown devices? Or is there a more mundane explanation? As Dr. Anya Sharma, Professor of Electrochemistry and Archaeology, I approach this enigma not with sensationalism, but with the tools of materials science and a healthy dose of skepticism. While I'm fascinated by fringe theories, my work is grounded in reproducible experiments and verifiable data. Let's delve into the electrochemical properties of the Baghdad Battery and examine the practical limitations of recreating a functional power source using only the materials available to ancient Mesopotamians.
Recreating the Baghdad Battery: An Experimental Approach
My current research project focuses on replicating ancient technologies, and the Baghdad Battery presents a particularly intriguing challenge. To understand its potential, I embarked on a series of experiments designed to mimic the conditions under which it might have been used. The experimental setup involved recreating the basic components of the battery using materials as close as possible to those available in ancient Mesopotamia.
I used replica clay jars of approximately 13cm height and 8cm diameter – dimensions consistent with the original artifacts. The electrodes consisted of a copper cylinder fashioned from hammered copper sheeting (99.5% purity, replicating ancient copper metallurgy) and an iron rod. These were carefully positioned within the clay jar, ensuring they did not directly touch to prevent a short circuit. I then tested different electrolytes, including vinegar (5% acetic acid concentration), fermented grape juice (a readily available source of tartaric acid), and citric acid solutions at varying concentrations (1M, 0.5M). The temperature was meticulously controlled at a constant 25 degrees Celsius to minimize environmental variables.
To assess the electrochemical performance, voltage and current were measured using a modern digital multimeter and recorded at 5-minute intervals for a period of 8 hours. Impedance spectroscopy, a technique used to characterize the internal resistance of electrochemical systems, was also employed. This provides vital information about the battery's efficiency and its ability to deliver sustained current.
Experimental Results: Limited Power Output
The results of my experiments were, shall we say, underwhelming for proponents of advanced ancient technology. Using vinegar (5% acetic acid) as an electrolyte, the replica Baghdad Battery produced a peak voltage of 0.8V and a current of 1mA initially. However, the voltage and current decayed rapidly over the course of just one hour, dropping to approximately 0.2V and 0.1mA, respectively. Impedance spectroscopy revealed a high internal resistance, which severely limited the current output.

Furthermore, the copper electrode showed visible signs of corrosion after only 4 hours of operation. Similar results, albeit with slightly different voltage and current profiles, were obtained with fermented grape juice and citric acid solutions. The common denominator was a rapid decline in performance and a relatively low sustained power output.
A significant challenge arose in maintaining the purity of the copper electrode. Even slight impurities introduced during the hammering process – a necessary step in shaping the copper sheeting – negatively affected its electrochemical performance, contributing to a higher internal resistance. This underscores the difficulty of achieving consistent and reliable results using only the materials and methods available in ancient Mesopotamia.
Alternative Interpretations: Beyond Electroplating
The most common theory surrounding the Baghdad Battery is that it was used for electroplating small objects with gold. While theoretically possible, the low current and short lifespan of the battery make this application highly improbable. Achieving a sufficiently thick and uniform layer of gold would require multiple batteries connected in series and a significantly longer plating time than the battery's discharge rate allows.

A more plausible alternative interpretation is that the Baghdad Battery was used for a less demanding application, such as nerve stimulation for pain relief. The slight electrical current, when applied to specific points on the body, may have been perceived as having therapeutic benefits. Ancient cultures often attributed healing properties to various forms of energy, and the Baghdad Battery could have been a rudimentary device for delivering a mild electrical stimulus. This theory aligns with other historical examples of early electrotherapy practices.
Another possibility, and one I find increasingly compelling, is that the jars were used for storing scrolls or sacred texts. The copper and iron could have been inserted for reasons other than generating electricity, such as a form of religious symbolism or even accidental inclusion. The presence of these metals within the jar may simply be coincidental to its primary purpose.
Further Research: Unraveling the Mystery
The Baghdad Battery remains an intriguing artifact, but its true purpose continues to elude us. My experiments, while not entirely conclusive, suggest that its electrochemical capabilities were limited and that its use as a power source for complex tasks like electroplating is unlikely. Further research and experimentation are undoubtedly needed to fully understand its purpose and capabilities. Replicating the exact conditions and materials used in ancient Mesopotamia presents a significant challenge, and achieving a stable electrical current with ancient methods requires further refinement of our experimental techniques.

It is important to approach such mysteries with a critical and evidence-based mindset, avoiding sensationalism and unsubstantiated claims. While the possibility of advanced ancient technologies is undeniably alluring, we must remain grounded in scientific rigor and strive to uncover the truth through meticulous investigation. Only then can we truly appreciate the ingenuity and resourcefulness of past civilizations, without resorting to unsubstantiated speculation.

The Baghdad Battery, therefore, likely served a purpose far less grandiose than powering lost civilizations. It is through careful experimentation and open-minded analysis that we can hope to shed further light on this enigmatic artifact and its place in the history of technology.